中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 陈敏玲, 韦献虎, 张菊梅, 丁郁, 吴清平. 2022
- CHEN Minling, WEI Xianhu, ZHANG Jumei, DING Yu, WU Qingping.
- 基于代谢组学的抗生素与细菌间作用研究进展
- Research progress of the interaction between antibiotics and bacteria based on metabolomics
- 微生物学报, 62(2): 403-413
- Acta Microbiologica Sinica, 62(2): 403-413
-
文章历史
- 收稿日期:2021-03-29
- 修回日期:2021-06-01
- 网络出版日期:2021-06-11
2. 华南理工大学生物科学与工程学院, 广东 广州 510006
2. College of Biological Sciences and Engineering, South China University of Technology, Guangzhou 510006, Guangdong, China
细菌耐药性严重威胁全球公共卫生,而新型抗生素开发进程缓慢,无法有效解决细菌耐药性问题[1]。由于抗生素在临床和养殖畜牧业的广泛应用,细菌耐药性呈迅速上升趋势,且抗生素抗性细菌和抗性基因可通过食物链在人群中进一步传播[2]。本团队在全国开展的食源性致病微生物风险调查表明多种食源性致病微生物对各种抗生素表现出不同程度的耐药性,如在1 024株食物来源的肠杆菌科分离株中,16.0%菌株对头孢菌素耐药,9.4%菌株具有超广谱β-内酰胺酶[3];且细菌多药耐药形势严峻,如75.3%水产品源蜡样芽胞杆菌对五种抗生素耐药[4]。面对严峻的细菌耐药情况,需加深对抗生素杀菌及细菌耐药机制的理解以改善现有杀菌策略。
目前抗生素可分为两类,一类是杀菌抗生素,包括氨基糖苷类、β-内酰胺类、喹诺酮类、糖肽类和脂肽类等;另一类是抑菌抗生素,包括大环内酯类、恶唑烷酮类、林可酰胺类、四环素类和氯霉素类等。当前关于抗生素与细菌代谢的研究主要集中在杀菌抗生素,除特殊说明,本综述所指抗生素均为杀菌抗生素。抗生素与靶点间相互作用已被广泛研究,对其下游事件深入研究发现不同作用机制的杀菌抗生素(氨苄西林、卡那霉素和诺氟沙星)均能够促进细菌产生羟基自由基从而加速细菌死亡[5]。研究表明细菌在抗生素作用下代谢发生显著变化,抗生素作用效果随其代谢状态变化而改变[6];且发现细菌代谢与抗性机制存在关联,如细胞壁降解和合成途径的中间代谢物能够诱导β-内酰胺酶的表达[7],表明细菌代谢积极参与在抗生素与细菌的相互作用过程中。
代谢组学是研究生物系统在特定条件下的整体代谢水平,它的应用已经从发现生物标志物拓展到理解生物表型背后的机制[8–9]。代谢组学通过监测细菌在抗生素作用下的代谢反应及适应机制,有利于揭示细菌代谢在抗生素杀菌过程中发挥的作用。基于代谢组学研究抗生素与细菌间相互作用有助于挖掘潜在代谢靶点,达到调控细菌在抗生素治疗下代谢反应、降低细菌生存率和细菌耐药性产生的目的[10]。
抗生素佐剂是指本身有很少或没有抗生素活性,但能增强现有抗生素活性或阻断耐药性产生的化合物。了解在抗生素杀菌过程中细菌的代谢变化[11]和细菌适应抗生素的代谢策略[12],有利于开发基于代谢物的抗生素佐剂以增强杀菌效果[13]或阻断细菌抗性产生[14],延长现有抗生素的使用寿命,缓解当前抗生素危机。
1 基于代谢组学研究抗生素作用机制抗生素已被成功地用于杀灭细菌,对抗生素与靶点作用后的下游事件进行深入研究,发现杀菌抗生素与靶点相互作用后,还通过改变细菌的代谢状态加速细菌死亡进程,如β-内酰胺类药物抑制其靶点青霉素结合蛋白(penicillin-binding proteins,PBPs)后,导致无效的细胞壁合成和降解循环,从而耗尽细胞资源,增强其杀菌活性[15];氨基糖苷类和氟喹诺酮类药物与其靶点作用后可引起脂质过氧化导致膜损伤,进而诱导细胞质凝聚加剧细菌死亡[16]。大肠杆菌经氨苄西林、卡那霉素或诺氟沙星处理后,胞内8-氧代鸟嘌呤水平均增加[11],在DNA复制过程中,8-氧代鸟嘌呤的掺入可导致DNA双链断裂,促进细菌死亡[17]。
Kohanski等于2007年提出不同作用机制的抗生素(氨苄西林、卡那霉素和诺氟沙星)作用于靶点后,细菌代谢随之发生全局变化,其中TCA循环被激活,NADH增加使电子传递链过度活化,刺激超氧化物(reactive oxygen species,ROS)形成,ROS进而破坏铁硫簇产生亚铁离子,亚铁离子与过氧化氢经芬顿反应氧化,产生羟基自由基破坏细胞内大分子,加速细胞死亡[5]。之后有研究对该机制提出不同意见[18–19],后续研究和综述对此提出详细验证和解释[20–21]。庆大霉素耐药溶藻弧菌和敏感溶藻弧菌的代谢组学分析表明,耐药菌株的糖酵解和丙酮酸循环减弱,ROS水平降低,外源添加葡萄糖通过促进菌株中心碳代谢、增加ROS水平,提高庆大霉素的杀菌能力[22],印证了ROS参与抗生素杀菌过程。
了解抗生素如何影响细菌代谢,代谢变化如何影响细菌生存能力,有利于治疗方案的优化。代谢组学为细菌代谢变化提供了直接的检测工具,大肠杆菌在氨苄西林、卡那霉素和诺氟沙星3种不同机制抗生素作用下的代谢组学研究发现,以上抗生素均引起还原型谷胱甘肽/氧化型谷胱甘肽比例下降,表明细菌经历持续的抗氧化反应;TCA循环活性增强,氧化磷酸化速率提高;核苷、核苷酸和嘌呤/嘧啶碱基水平显著下降,可能由DNA损伤、核苷酸周转加速导致;毒性代谢副产物产生,双链DNA断裂,该结果说明细菌在抗生素作用下经历细胞损伤和功能失调,并最终死亡[11]。对大肠杆菌在不同浓度喹诺酮类药物作用下的代谢组学进行研究,发现喹诺酮作用于促旋酶后,(dTDP)-鼠李糖上调最显著,(dTDP)-鼠李糖可作为促旋酶活性的指标,促旋酶GyrA亚基的表达由呼吸代谢转录因子ArcA过表达所诱导,由此揭示细菌代谢变化与DNA复制的协调关系[8]。
2 基于代谢组学研究细菌耐药机制 2.1 抗生素失效与细菌代谢的联系抗生素治疗可显著改变细菌的代谢状态,反之,细菌代谢状态也影响其对抗生素的敏感性。抗生素治疗失败由细菌耐药性、耐受性和持留性导致[23]。耐药性指细菌群体在抗生素作用下具有生存和复制的能力,可通过基因遗传、突变、水平转移获得。耐受性[24]是指细菌群体没有耐药基因,但能在杀菌浓度的抗生素下暂时存活。持留性[25]是指细菌中小部分群体能在杀菌浓度的抗生素下存活。
耐药细菌除了通过经典抗性机制获得耐药能力,还可能通过代谢适应改变其代谢状态以减轻抗生素的毒性,在代谢进化实验中,大肠杆菌编码2-酮戊二酸脱氢酶的sucA基因发生突变,其整体代谢活性下调、呼吸作用减弱,因而对羧苄青霉素的抗性显著增加,该突变基因具有临床相关性[26]。此外,细菌代谢也参与到细菌耐药机制的调控过程中。多药外排泵不仅负责药物的外排,也影响代谢物的进出,相关代谢底物可对外排泵进行反馈调控,大肠杆菌肠杆菌素合成途径中的2, 3-二羟基苯甲酸酯通过与转录因子MarR结合,激活marRAB转录,进而影响外排泵AcrAB-TolC的表达[27]。β-内酰胺酶通过降解β-内酰胺类药物使菌株耐药,β-内酰胺酶的表达受细胞壁降解和合成途径中代谢物调控,在革兰氏阴性菌中,尿苷二磷酸-N-乙酰胞壁酸-五肽(UDP-MurNAc- pentapeptide)、1, 6-脱水乙酰胞壁酸-三肽(1, 6-anhMurNAc-tripeptide)等参与ampC β-内酰胺酶的表达调控[7];革兰氏阳性菌地衣芽胞杆菌通过γ-D-Glu-m-A2pm二肽与BlaI抑制子结合,激活β-内酰胺酶BlaZ的表达,金黄色葡萄球菌通过γ-D-Glu-L-Lys二肽激活mecA基因表达PBP2a[28],该蛋白对β-内酰胺类药物亲和力低。
细菌获得药物抗性后,生长代谢也发生了相应变化,使用代谢组学对抗性细菌进行代谢分析,可系统了解细菌代谢如何影响抗生素疗效。多粘菌素是抵御多药耐药革兰氏阴性菌感染的最后一道防线,质粒介导的多粘菌素耐药基因mcr-1已在多种肠杆菌中被鉴定出来[29],对公共卫生构成了巨大挑战。本实验室首次在肠杆菌科外的副溶血性弧菌中发现mcr-1,表明mcr-1具有潜在转移风险[30]。mcr-1的表达使大肠杆菌获得多粘菌素抗性,同时造成适应性代价,通过代谢组学发现过表达mcr-1的大肠杆菌脂质代谢增强,核苷酸和TCA循环水平降低[31]。大肠杆菌过表达AcrAB外排泵获得对氯霉素抗性,其代谢转变为发酵状态,对氨苄西林耐药的大肠杆菌进行代谢组学分析,发现其核苷酸代谢、丝氨酸生物合成和细胞壁循环途径均发生变化[10]。对哌拉西林耐药的铜绿假单胞菌缺失了分解亮氨酸的能力,该表型在临床菌株中得到印证[32]。
耐受菌和持留菌代谢状态相似,均表现为生长缓慢,早期研究发现非生长细菌对药物不敏感,其生长率和抗生素致死性呈正相关关系,在临床中也常发现生长缓慢难以根除的细菌[12]。除了生长速率这个代谢指标,近年来发现细菌代谢活性与抗生素效果也存在联系。结核分枝杆菌在缺氧环境下,代谢通量从TCA循环转向甘油三酯合成,生长和代谢活性降低,对抗生素耐受能力增强[12]。大肠杆菌转酮醇酶A基因(tktA)和3-磷酸甘油脱氢酶基因(glpD)的无效突变体可以增加菌株的持留性,该过程的已知机制是通过改变代谢流量增加生长抑制代谢物丙酮醛产生[33]。金黄色葡萄球菌在指数生长期时,部分细菌随机进入稳定期,通过降低胞内ATP水平形成持留菌,提高对抗生素的抵抗能力[34]。
2.2 碳代谢碳代谢为细菌提供主要能量和其他途径的前体,该代谢途径已被证明影响多种细菌对抗生素的敏感性。本实验室发现耐头孢西丁创伤弧菌的蛋白发生大量乙酰化修饰,发生修饰的蛋白主要富集在碳水化合物代谢和能量代谢途径,表明代谢调节对细菌耐药有潜在影响[35]。结核分枝杆菌在异烟肼、利福平或链霉素作用下,均上调具有氧化保护作用的异柠檬酸分解酶,同时,基于代谢组学发现该菌代谢状态表现为TCA循环流量减少,乙醛酸分流活性增加,呼吸作用减弱[36],表明细菌对药物耐受可能是一个主动反应的生理过程,而不是被动的生长缓慢或停滞。对万古霉素中介耐药和敏感菌进行比较代谢组学分析,发现中介耐药菌减少TCA循环通量,增加嘌呤合成、PEP途径和细胞壁合成通量,外加嘌呤合成和PEP途径抑制剂均增加万古霉素对金黄色葡萄球菌的杀灭作用[14]。
碳代谢途径对氨基糖苷类药物的影响被研究得较为清楚。通过代谢组学发现耐卡那霉素的爱德华氏菌胞内葡萄糖和丙氨酸丰度显著降低,外源添加葡萄糖或丙氨酸能够恢复卡那霉素对耐药细菌的杀菌能力[37]。这是由于氨基糖苷类抗生素需要质子动力(proton motive force,PMF)的转运才能进入细胞发挥杀菌功能,而TCA循环是PMF的主要来源,外源添加的葡萄糖或丙氨酸通过激活TCA循环增加NADH产生,NADH激活电子传递链促进PMF产生,从而增加细菌对抗生素的摄取,使杀菌效果增强[37]。外源添加谷氨酸也能增强卡那霉素对耐药大肠杆菌的杀菌能力,谷氨酸通过丙酮酸循环进入TCA循环后,通过上述机制促进细菌代谢活性从而增强杀菌效果[38]。外加TCA循环中间代谢物富马酸能够增强妥布霉素对铜绿假单胞菌持留菌的杀伤能力,该机制可能是氨基糖苷类抗生素特有的,富马酸不影响β-内酰胺类和喹诺酮类抗生素的效果[39]。
发生在耐药菌株上的基因突变也表明了碳代谢对细菌耐药表型的影响,如表皮葡萄球菌临床分离株中的TCA循环缺陷株对β-内酰胺类药物显示出更强的耐受性[40]。编码酮戊二酸脱氢酶的sucA或sucB基因失活能够增加大肠杆菌在环丙沙星下的存活率[41]。对大肠杆菌突变体库进行筛选,发现细胞壁合成、核苷酸代谢和碳水化合物代谢功能缺失的菌株对环丙沙星表现出更强的敏感性[42]。
2.3 细胞呼吸大多数抗生素作用的途径与能量消耗相关,研究发现细胞呼吸和能量代谢对抗生素杀菌能力也具有重要影响。细菌在大部分杀菌抗生素作用下表现为呼吸作用增强,从而加速抗生素杀菌进程,在抑菌抗生素作用下表现为呼吸减缓,当杀菌抗生素和抑菌抗生素联合使用时,抑菌抗生素对细菌的呼吸抑制效果占主导,阻断杀菌抗生素对细菌代谢活动的激活,从而降低杀菌抗生素杀菌效果[43]。
通过代谢组学对头孢他啶耐药和敏感的溶藻弧菌进行分析,发现耐药菌株呼吸效率和PMF降低,丙酮酸循环下调,TCA循环和丙酮酸循环抑制剂的加入使耐药菌株抗性进一步增强[44]。铜绿假单胞菌在生物膜缺氧区域中缺少电子受体氧气,其生产吩嗪作为电子受体以替代原本的氧化还原平衡策略,该能量代谢状态赋予其对环丙沙星的耐受性[45]。大肠杆菌和金黄色葡萄球菌经敲除细胞色素氧化酶后,呼吸作用被抑制,对抗生素的耐受性随之增强[43]。亚硝酸钠作为抗菌剂对细胞呼吸有抑制作用,其同样增强了铜绿假单胞菌对环丙沙星的耐受能力[46]。
在研究妥布霉素杀灭铜绿假单胞菌的实验中,外源添加富马酸使细菌代谢活性增强,产生更多还原电子载体提供给电子传递链,进而产生更多PMF,增加细菌对妥布霉素的摄取;而外加乙醛酸使细菌的代谢通量从TCA循环中分流出来,细胞呼吸被抑制,减弱了妥布霉素的杀菌效果;当同时加入富马酸和乙醛酸时,即使细菌体内存在妥布霉素,乙醛酸诱导的药物耐受表型仍占主导,这表明药物摄取不足以说明细菌死亡的原因,下游代谢反应及呼吸作用对药物杀菌起着重要作用[39]。
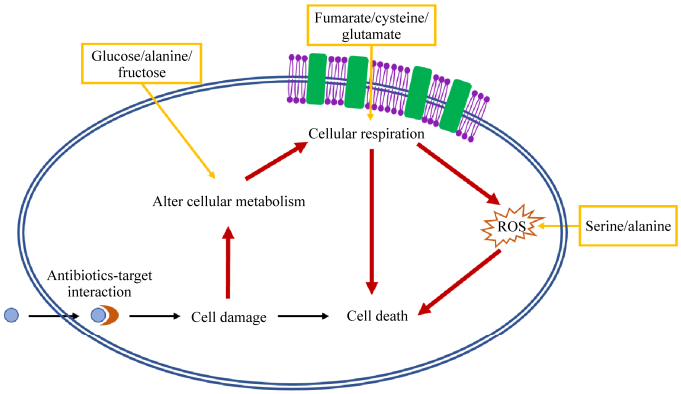
3 挖掘基于代谢物的抗生素佐剂细菌调节其代谢以适应抗生素压力(图 1),因此通过代谢组学研究抗生素作用及细菌耐药机制,有利于挖掘抗生素佐剂,以调控细菌代谢而改变抗生素效果(表 1)。基于代谢物的抗生素佐剂可用于提高氨基糖苷类药物的杀菌能力,氨基糖苷类药物和糖酵解途径中间代谢物(葡萄糖、果糖、甘露醇或丙酮酸)的组合使大肠杆菌持留菌生存能力降低3个数量级;不过在同样的实验条件下,只有果糖可以增强庆大霉素对金黄色葡萄球菌持留菌的杀灭效果[13],表明该策略需根据不同病原菌进行调整。氨基糖苷类药物杀菌作用得到增强是由于外加的代谢物激活电子传递链,增加PMF,从而增加细菌对药物的摄取。PMF由Δψ (跨膜电势)和ΔpH (跨膜H+浓度梯度)组成[47],上述策略是通过激活电子传递链增加跨膜电势从而增加PMF,而外加碱性氨基酸精氨酸可以通过改变H+浓度增加庆大霉素对大肠杆菌、金黄色葡萄球菌和铜绿假单胞菌的杀菌效果[48]。细菌耐药存在多种机制,该方法可能对某些耐药机制无效,例如药物修饰酶等,因此需更全面地研究以抵抗细菌不同的耐药策略。
|
| 图 1 细菌代谢影响抗生素杀菌作用 Figure 1 Effect of bacterial metabolism on antibiotic efficacy. Interactions between antibiotic and target trigger cell death (black). The perturbations of downstream cellular metabolism, respiration and ROS levels contribute to the death of bacteria (red). The additions of metabolic adjuvants enhance the antibiotic efficacy by acting on cellular metabolism, respiration or ROS production (yellow). |
| Metabolites | Antibiotics | Effect on antibiotics | Bacteria | Mechanisms | References |
| L-serine | Fluoroquinolone | Potentiate | E. coli | Enhances endogenous ROS production | [51] |
| Glucose, mannitol or pyruvate | Gentamicin | Potentiate | E. coli | Increased PMF stimulates uptake of antibiotic | [13] |
| L-arginine | Gentamicin | Potentiate | S. aureus, E. coli and P. aeruginosa | Alters PMF by modifying the membrane pH gradient | [48] |
| Fumarate | Tobramycin | Potentiate | P. aeruginosa | Activates cellular respiration and generates PMF by stimulating TCA cycle | [39] |
| Glucose and fumarate | Ciprofloxacin | Potentiate | E. coli | Increases cellular respiration | [49] |
| Cysteine | Isoniazid | Potentiate | M. tuberculosis | Increases cellular respiration and ROS production | [50] |
| Glyoxylate | Tobramycin | Decrease | P. aeruginosa | Inhibits cellular respiration through glyoxylate shunt | [39] |
| Adenine | Ampicillin and ciprofloxacin | Decrease | E. coli | Rescues antibiotic-induced purine depletion and reduces cellular respiration | [9] |
除了氨基糖苷类药物与代谢物协同杀菌,喹诺酮类药物与葡萄糖和富马酸组合[49]、异烟肼和半胱氨酸组合[50]也能通过增强细菌代谢活性和呼吸作用提高药物的杀菌效果。丝氨酸和喹诺酮类药物组合提高了后者对大肠杆菌持留菌和临床抗性菌株的杀灭效果,该策略通过增加NAD+/NADH,破坏铁硫簇,进而增加ROS水平,提高杀菌作用[51]。提高细菌ROS水平显著增加β-内酰胺类和喹诺酮类抗生素的杀菌活性,但未能增强氨基糖苷类抗生素效果,因为ROS水平增加影响PMF,从而限制细菌对药物的摄取[52]。
代谢组学除了为抗生素与细菌间作用提供直接和准确的代谢见解,也为抗生素佐剂的理性开发提供指导。对大肠杆菌进行代谢组学分析,发现其在氨苄西林或环丙沙星作用下胞内游离腺嘌呤快速耗竭,为恢复核苷酸稳态,胞内ATP合成、中心碳代谢活性和耗氧量增加,进而加剧抗生素的杀灭作用,外源添加嘧啶能够抑制嘧啶合成、激活嘌呤合成,达到增强抗生素杀菌作用的效果[9]。代谢组学分析表明贝达喹啉除了抑制结核分枝杆菌的ATP合酶,还显著抑制谷氨酰胺合成酶,外加谷氨酰胺合成酶抑制剂能有效增强贝达喹啉的杀菌作用[53]。
4 总结与展望细菌代谢与抗生素效果的深入研究拓宽了以往抗生素与靶点相互作用的观点,将细菌整体代谢过程与抗生素杀菌联系起来。目前研究表明细菌代谢积极参与抗生素杀菌过程中,抗生素杀菌效果受细菌代谢状态影响,外源添加代谢物能够调节抗生素杀菌效果。代谢组学为系统解析抗生素与细菌相互作用过程的代谢变化提供了有力工具,可用于揭示细菌在抗生素作用下的代谢变化、耐药细菌和敏感细菌的代谢差异[9, 14],探究细菌在感知抗生素、部署防线、死亡或存活过程中的代谢变化,为合理设计和改善治疗方案提供理论依据。此外,代谢组学还可用于推断抗菌化合物的作用机制,挖掘作用机制未知的抗生素[54],促进新型抗生素的发现。
随着组学技术的发展,多组学联用为研究细菌代谢和抗生素作用间关系提供了全面和系统的理论支持[42, 55–56]。转录组学、蛋白质组学和代谢组学的整合分析发现乙酸通过激活苯丙氨酸降解途径中phhA、hmgA和hpd基因,增强Ga(III)的抑菌活性[55];对基因组学和代谢组学进行整合有利于明晰代谢基因单核苷酸多态性对细菌药物敏感表型的影响[56];基因组规模代谢模型与代谢组学相结合有助于快速挖掘调节抗生素效果的代谢物[9]。多组学整合分析为细菌代谢和抗生素杀菌的联系提供深刻见解,加速抗生素佐剂的挖掘进程。
在实际应用中,宿主体内的代谢物与抗生素存在相加、协同或拮抗等相互作用,需明确解析宿主体内代谢环境对抗生素效果的影响,以完成抗生素佐剂应用的落地。在感染大肠杆菌的小鼠模型中,抗生素在感染部位诱导多种代谢物的变化,其中上调的一磷酸腺苷降低抗生素效果,增强免疫细胞吞噬活性,但抗生素直接作用于免疫细胞会抑制其呼吸作用,从而削弱其吞噬活性[57]。受金黄色葡萄球菌感染的小鼠体内亮氨酸、脯氨酸、异亮氨酸和缬氨酸显著增加,外源加入这些氨基酸能够抑制精氨酸酶活性,促进巨噬细胞产生NO,进而增强其杀菌能力[58]。
我们对细菌代谢与抗生素杀菌效果间联系的理解还处于起步阶段,更透彻地了解细菌在抗生素下死亡的代谢机制、耐药细菌产生抗性的代谢基础和耐受细菌的代谢调节机制,能够帮助我们克服细菌的防御机制,实现高效而精准的抗菌治疗。
| [1] | Piddock LJ. The crisis of no new antibiotics—what is the way forward? The Lancet Infectious Diseases, 2012, 12(3): 249–253. |
| [2] | Muloi D, Ward MJ, Pedersen AB, Fèvre EM, Woolhouse MEJ, Van Bunnik BAD. Are food animals responsible for transfer of antimicrobial-resistant Escherichia coli or their resistance determinants to human populations? A systematic review. Foodborne Pathogens and Disease, 2018, 15(8): 467-474. DOI:10.1089/fpd.2017.2411 |
| [3] | Ye QH, Wu QP, Zhang SH, Zhang JM, Yang GZ, Wang J, Xue L, Chen MT. Characterization of extended-spectrum β-lactamase-producing Enterobacteriaceae from retail food in China. Frontiers in Microbiology, 2018, 9: 1709. DOI:10.3389/fmicb.2018.01709 |
| [4] | Zhang Y, Chen MF, Yu PF, Yu SB, Wang J, Guo H, Zhang JH, Zhou H, Chen MT, Zeng HY, Wu S, Pang R, Ye QH, Xue L, Zhang SH, Li Y, Zhang JM, Wu QP, Ding Y. Prevalence, virulence feature, antibiotic resistance and MLST typing of Bacillus cereus isolated from retail aquatic products in China. Frontiers in Microbiology, 2020, 11: 1513. DOI:10.3389/fmicb.2020.01513 |
| [5] | Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell, 2007, 130(5): 797-810. DOI:10.1016/j.cell.2007.06.049 |
| [6] | Stokes JM, Lopatkin AJ, Lobritz MA, Collins JJ. Bacterial metabolism and antibiotic efficacy. Cell Metabolism, 2019, 30(2): 251-259. DOI:10.1016/j.cmet.2019.06.009 |
| [7] | Dik DA, Fisher JF, Mobashery S. Cell-wall recycling of the Gram-negative bacteria and the nexus to antibiotic resistance. Chemical Reviews, 2018, 118(12): 5952-5984. DOI:10.1021/acs.chemrev.8b00277 |
| [8] | Zampieri M, Zimmermann M, Claassen M, Sauer U. Nontargeted metabolomics reveals the multilevel response to antibiotic perturbations. Cell Reports, 2017, 19(6): 1214-1228. DOI:10.1016/j.celrep.2017.04.002 |
| [9] | Yang JH, Wright SN, Hamblin M, McCloskey D, Alcantar MA, Schrübbers L, Lopatkin AJ, Satish S, Nili A, Palsson BO, Walker GC, Collins JJ. A white-box machine learning approach for revealing antibiotic mechanisms of action. Cell, 2019, 177(6): 1649-1661.e9. DOI:10.1016/j.cell.2019.04.016 |
| [10] | Zampieri M, Enke T, Chubukov V, Ricci V, Piddock L, Sauer U. Metabolic constraints on the evolution of antibiotic resistance. Molecular Systems Biology, 2017, 13(3): 917. DOI:10.15252/msb.20167028 |
| [11] | Belenky P, Ye JD, Porter CBM, Cohen NR, Lobritz MA, Ferrante T, Jain S, Korry BJ, Schwarz EG, Walker GC, Collins JJ. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Reports, 2015, 13(5): 968-980. DOI:10.1016/j.celrep.2015.09.059 |
| [12] | Baek SH, Li AH, Sassetti CM. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biology, 2011, 9(5): e1001065. DOI:10.1371/journal.pbio.1001065 |
| [13] | Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature, 2011, 473(7346): 216-220. DOI:10.1038/nature10069 |
| [14] | Gardner SG, Marshall DD, Daum RS, Powers R, Somerville GA. Metabolic mitigation of Staphylococcus aureus vancomycin intermediate-level susceptibility. Antimicrobial Agents and Chemotherapy, 2018, 62(1): e01608-e01617. |
| [15] | Cho H, Uehara T, Bernhardt TG. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell, 2014, 159(6): 1300-1311. DOI:10.1016/j.cell.2014.11.017 |
| [16] | Wong F, Stokes JM, Cervantes B, Penkov S, Friedrichs J, Renner LD, Collins JJ. Cytoplasmic condensation induced by membrane damage is associated with antibiotic lethality. Nature Communications, 2021, 12: 2321. DOI:10.1038/s41467-021-22485-6 |
| [17] | Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science, 2012, 336(6079): 315-319. DOI:10.1126/science.1219192 |
| [18] | Liu YY, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science, 2013, 339(6124): 1210-1213. DOI:10.1126/science.1232751 |
| [19] | Keren I, Wu YX, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science, 2013, 339(6124): 1213-1216. DOI:10.1126/science.1232688 |
| [20] | Dwyer DJ, Collins JJ, Walker GC. Unraveling the physiological complexities of antibiotic lethality. Annual Review of Pharmacology and Toxicology, 2015, 55: 313-332. DOI:10.1146/annurev-pharmtox-010814-124712 |
| [21] | Van Acker H, Coenye T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends in Microbiology, 2017, 25(6): 456-466. DOI:10.1016/j.tim.2016.12.008 |
| [22] | Zhang S, Yang MJ, Peng B, Peng XX, Li H. Reduced ROS-mediated antibiotic resistance and its reverting by glucose in Vibrio alginolyticus. Environmental Microbiology, 2020, 22(10): 4367-4380. DOI:10.1111/1462-2920.15085 |
| [23] | Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo JM, Hardt WD, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan MW, Tenson T, Van Melderen L, Zinkernagel A. Definitions and guidelines for research on antibiotic persistence. Nature Reviews Microbiology, 2019, 17(7): 441-48. DOI:10.1038/s41579-019-0196-3 |
| [24] | Windels EM, Michiels JE, Van Den Bergh B, Fauvart M, Michiels J. Antibiotics: combatting tolerance to stop resistance. mBio, 2019, 10(5): e02095-19. |
| [25] | Jung SH, Ryu CM, Kim JS. Bacterial persistence: fundamentals and clinical importance. Journal of Microbiology, 2019, 57(10): 829-835. DOI:10.1007/s12275-019-9218-0 |
| [26] | Lopatkin AJ, Bening SC, Manson AL, Stokes JM, Kohanski MA, Badran AH, Earl AM, Cheney NJ, Yang JH, Collins JJ. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science, 2021, 371(6531): eaba0862. DOI:10.1126/science.aba0862 |
| [27] | Chubiz LM, Rao CV. Aromatic acid metabolites of Escherichia coli K-12 can induce the marRAB operon. Journal of Bacteriology, 2010, 192(18): 4786-4789. DOI:10.1128/JB.00371-10 |
| [28] | Amoroso A, Boudet J, Berzigotti S, Duval V, Teller N, Mengin-Lecreulx D, Luxen A, Simorre JP, Joris B. A peptidoglycan fragment triggers β-lactam resistance in Bacillus licheniformis. PLoS Pathogens, 2012, 8(3): e1002571. DOI:10.1371/journal.ppat.1002571 |
| [29] | Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian GB, Dong BL, Huang XH, Yu LF, Gu DX, Ren HW, Chen XJ, Lv L, He DD, Zhou HW, Liang ZS, Shen JZ. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious Diseases, 2016, 16(2): 161-168. DOI:10.1016/S1473-3099(15)00424-7 |
| [30] | Lei T, Zhang JM, Jiang FF, He M, Zeng HY, Chen MT, Wu S, Wang J, Ding Y, Wu QP. First detection of the plasmid-mediated colistin resistance gene mcr-1 in virulent Vibrio parahaemolyticus. International Journal of Food Microbiology, 2019, 308: 108290. DOI:10.1016/j.ijfoodmicro.2019.108290 |
| [31] | Liu YY, Zhu Y, Wickremasinghe H, Bergen PJ, Lu J, Zhu XQ, Zhou QL, Azad M, Nang SC, Han ML, Lei T, Li J, Liu JH. Metabolic perturbations caused by the over-expression of mcr-1 in Escherichia coli. Frontiers in Microbiology, 2020, 11: 588658. DOI:10.3389/fmicb.2020.588658 |
| [32] | Dunphy LJ, Yen P, Papin JA. Integrated experimental and computational analyses reveal differential metabolic functionality in antibiotic-resistant Pseudomonas aeruginosa. Cell Systems, 2019, 8(1): 3-14.e3. DOI:10.1016/j.cels.2018.12.002 |
| [33] | Girgis HS, Harris K, Tavazoie S. Large mutational target size for rapid emergence of bacterial persistence. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(31): 12740-12745. DOI:10.1073/pnas.1205124109 |
| [34] | Conlon BP, Rowe SE, Gandt AB, Nuxoll AS, Donegan NP, Zalis EA, Clair G, Adkins JN, Cheung AL, Lewis K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nature Microbiology, 2016, 1: 16051. DOI:10.1038/nmicrobiol.2016.51 |
| [35] | Pang R, Li Y, Liao K, Guo PH, Li YP, Yang XJ, Zhang SH, Lei T, Wang J, Chen MT, Wu S, Xue L, Wu QP. Genome- and proteome-wide analysis of lysine acetylation in Vibrio vulnificus Vv180806 reveals its regulatory roles in virulence and antibiotic resistance. Frontiers in Microbiology, 2020, 11: 591287. DOI:10.3389/fmicb.2020.591287 |
| [36] | Nandakumar M, Nathan C, Rhee KY. Isocitrate lyase mediates broad antibiotic tolerance in Mycobacterium tuberculosis. Nature Communications, 2014, 5: 4306. DOI:10.1038/ncomms5306 |
| [37] | Peng B, Su YB, Li H, Han Y, Guo C, Tian YM, Peng XX. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metabolism, 2015, 21(2): 249-262. DOI:10.1016/j.cmet.2015.01.008 |
| [38] | Su YB, Peng B, Li H, Cheng ZX, Zhang TT, Zhu JX, Li D, Li MY, Ye JZ, Du CC, Zhang S, Zhao XL, Yang MJ, Peng XX. Pyruvate cycle increases aminoglycoside efficacy and provides respiratory energy in bacteria. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(7): E1578-E1587. DOI:10.1073/pnas.1714645115 |
| [39] | Meylan S, Porter CBM, Yang JH, Belenky P, Gutierrez A, Lobritz MA, Park J, Kim SH, Moskowitz SM, Collins JJ. Carbon sources tune antibiotic susceptibility in Pseudomonas aeruginosa via tricarboxylic acid cycle control. Cell Chemical Biology, 2017, 24(2): 195-206. DOI:10.1016/j.chembiol.2016.12.015 |
| [40] | Thomas VC, Kinkead LC, Janssen A, Schaeffer CR, Woods KM, Lindgren JK, Peaster JM, Chaudhari SS, Sadykov M, Jones J, AbdelGhani SM, Zimmerman MC, Bayles KW, Somerville GA, Fey PD. A dysfunctional tricarboxylic acid cycle enhances fitness of Staphylococcus epidermidis during β-lactam stress. mBio, 2013, 4(4): e00437-13. |
| [41] | Wang Y, Bojer MS, George SE, Wang ZH, Jensen PR, Wolz C, Ingmer H. Inactivation of TCA cycle enhances Staphylococcus aureus persister cell formation in stationary phase. Scientific Reports, 2018, 8: 10849. DOI:10.1038/s41598-018-29123-0 |
| [42] | Stokes JM, Gutierrez A, Lopatkin AJ, Andrews IW, French S, Matic I, Brown ED, Collins JJ. A multiplexable assay for screening antibiotic lethality against drug-tolerant bacteria. Nature Methods, 2019, 16(4): 303-306. DOI:10.1038/s41592-019-0333-y |
| [43] | Lobritz MA, Belenky P, Porter CBM, Gutierrez A, Yang JH, Schwarz EG, Dwyer DJ, Khalil AS, Collins JJ. Antibiotic efficacy is linked to bacterial cellular respiration. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(27): 8173-8180. DOI:10.1073/pnas.1509743112 |
| [44] | Liu SR, Peng XX, Li H. Metabolic mechanism of ceftazidime resistance in Vibrio alginolyticus. Infection and Drug Resistance, 2019, 12: 417-429. DOI:10.2147/IDR.S179639 |
| [45] | Schiessl KT, Hu FH, Jo J, Nazia SZ, Wang B, Price-Whelan A, Min W, Dietrich LEP. Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nature Communications, 2019, 10: 762. DOI:10.1038/s41467-019-08733-w |
| [46] | Zemke AC, Kocak BR, Bomberger JM. Sodium nitrite inhibits killing of Pseudomonas aeruginosa biofilms by ciprofloxacin. Antimicrobial Agents and Chemotherapy, 2017, 61(1): e00448-e00416. |
| [47] | Mates SM, Eisenberg ES, Mandel LJ, Patel L, Kaback HR, Miller MH. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proceedings of the National Academy of Sciences of the United States of America, 1982, 79(21): 6693-6697. DOI:10.1073/pnas.79.21.6693 |
| [48] | Lebeaux D, Chauhan A, Létoffé S, Fischer F, De Reuse H, Beloin C, Ghigo JM. pH-mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms. The Journal of Infectious Diseases, 2014, 210(9): 1357-1366. DOI:10.1093/infdis/jiu286 |
| [49] | Gutierrez A, Jain S, Bhargava P, Hamblin M, Lobritz MA, Collins JJ. Understanding and sensitizing density-dependent persistence to quinolone antibiotics. Molecular Cell, 2017, 68(6): 1147-1154.e3. DOI:10.1016/j.molcel.2017.11.012 |
| [50] | Vilchèze C, Hartman T, Weinrick B, Jain P, Weisbrod TR, Leung LW, Freundlich JS, Jacobs WR. Enhanced respiration prevents drug tolerance and drug resistance in Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(17): 4495-4500. DOI:10.1073/pnas.1704376114 |
| [51] | Duan XK, Huang X, Wang XY, Yan SQ, Guo SY, Abdalla AE, Huang CW, Xie JP. L-serine potentiates fluoroquinolone activity against Escherichia coli by enhancing endogenous reactive oxygen species production. The Journal of Antimicrobial Chemotherapy, 2016, 71(8): 2192-2199. DOI:10.1093/jac/dkw114 |
| [52] | Brynildsen MP, Winkler JA, Spina CS, MacDonald IC, Collins JJ. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nature Biotechnology, 2013, 31(2): 160-165. DOI:10.1038/nbt.2458 |
| [53] | Wang Z, Soni V, Marriner G, Kaneko T, Boshoff HIM, Barry CE 3rd, Rhee KY. Mode-of-action profiling reveals glutamine synthetase as a collateral metabolic vulnerability of M. tuberculosis to bedaquiline. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(39): 19646-19651. DOI:10.1073/pnas.1907946116 |
| [54] | Zampieri M, Szappanos B, Buchieri MV, Trauner A, Piazza I, Picotti P, Gagneux S, Borrell S, Gicquel B, Lelievre J, Papp B, Sauer U. High-throughput metabolomic analysis predicts mode of action of uncharacterized antimicrobial compounds. Science Translational Medicine, 2018, 10(429): eaal3973. DOI:10.1126/scitranslmed.aal3973 |
| [55] | Wang YC, Han BJ, Xie YX, Wang HB, Wang RM, Xia W, Li HY, Sun HZ. Combination of gallium(III) with acetate for combating antibiotic resistant Pseudomonas aeruginosa. Chemical Science, 2019, 10(24): 6099-6106. DOI:10.1039/C9SC01480B |
| [56] | Øyås O, Borrell S, Trauner A, Zimmermann M, Feldmann J, Liphardt T, Gagneux S, Stelling J, Sauer U, Zampieri M. Model-based integration of genomics and metabolomics reveals SNP functionality in Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(15): 8494-8502. DOI:10.1073/pnas.1915551117 |
| [57] | Yang JH, Bhargava P, McCloskey D, Mao N, Palsson BO, Collins JJ. Antibiotic-induced changes to the host metabolic environment inhibit drug efficacy and alter immune function. Cell Host & Microbe, 2017, 22(6): 757-765.e3. |
| [58] | Pang R, Zhou H, Huang YF, Su YB, Chen XH. Inhibition of host arginase activity against staphylococcal bloodstream infection by different metabolites. Frontiers in Immunology, 2020, 11: 1639. DOI:10.3389/fimmu.2020.01639 |
 2022, Vol. 62
2022, Vol. 62