中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 李平, 谭添, 刘韩, 王和林. 2021
- Li Ping, Tan Tian, Liu Han, Wang Helin. 2021
- 地下水微生物功能群及生物地球化学循环
- Functional microbial communities and the biogeochemical cycles in groundwater
- 微生物学报, 61(6): 1598-1609
- Acta Microbiologica Sinica, 61(6): 1598-1609
-
文章历史
- 收稿日期:2020-10-04
- 修回日期:2021-01-25
- 网络出版日期:2021-02-24
地下水系统是地球关键带的重要组成部分,是连接地表生物圈-深部地下生物圈的桥梁和纽带。世界范围内有超过三分之二的饮用水来自地下水,我国60%以上的城市开采利用地下水[1-2]。随着人类活动日益频繁,地下水污染逐渐加剧,地下水可持续安全供给受到空前挑战。微生物是地下水生态系统的重要组成部分,是地下水系统物质循环和能量流动的控制器。地下水低温、缺氧、低有机质和黑暗等特点为微生物提供了特殊的栖息环境[3];地下水系统的非均质性和水文地质过程的非线性又为微生物提供了极其复杂的生存条件,进而演化出复杂的微生物功能群和生物地球化学过程。开展地下水微生物功能群及生物地球化学循环的研究有助于我们深入了解地下水水质的演化过程,为地下水资源保护和地下水污染修复提供科学依据。
随着科学技术的不断革新,近年来地下水微生物功能群及生物地球化学过程的研究呈现快速增长态势。特别是随着近10年来多学科技术的飞速发展,分子生物学、多组学、同位素、地球化学分析和数值模拟等多种技术方法、多学科的交叉融合与应用,地下水微生物功能群及生物地球化学循环研究取得了一系列引人瞩目的重要进展,主要有以下几个方面:(1) 地下水微生物功能群及功能分区;(2) 地下水微生物介导的地球化学元素循环;(3) 地下水污染与修复的生物地球化学过程;(4) 地下水生物地球化学过程数值模拟。本文将从这些方面对近年来国内外相关研究进展进行综述,并对其未来发展方向进行展望。
1 主要研究进展 1.1 地下水系统中微生物功能群及功能分区地下水环境复杂的地球化学条件为微生物提供了多样的栖息环境,进而演化出了复杂的微生物功能群。研究地下水中的微生物功能群及其相互影响,能够更清楚地了解氧化还原条件变化过程中微生物群落的演替,为厘清地下水微生物与地球化学的协同关系提供依据。
有研究表明,地球上约40%的原核生物都栖息在地下含水层这一特殊环境中[4],大量的微生物新种群还未被发现。研究者们通过16S rRNA扩增子测序和宏基因组学等技术对多个国家的地下水水体和沉积物以及岩溶地下水中的微生物群落及其功能进行了研究,发现了惊人的微生物多样性以及大量的新种;微生物介导的碳、氮、硫循环代谢活动相互依存,呈“流水线”式有序协作完成[5-7];地下水微生物菌群功能多样,几乎占据了所有可能的菌群生态位。目前发现的地下水微生物可能参与有机物降解、产甲烷和甲烷氧化、硫氧化还原、铁氧化还原、氨化、固氮、硝化和反硝化等多种代谢活动[8-10]。
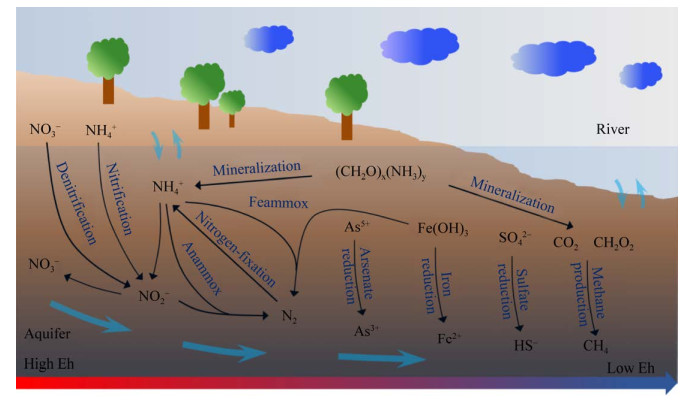
由于地下水流速缓慢,微生物长期累积并作用于碳、氮、铁、硫等元素的循环和迁移转化,从而改变了地下水的化学组分以及氧化还原电位(Eh)。从地下水补给区到排泄区,随着Eh的逐渐降低,地下水系统呈现了不同的生态位以及微生物功能群分区。这些微生物功能群在不同的Eh条件下分别进行有机物分解、硝酸盐还原、铁还原、硫酸盐还原和产甲烷等过程[11-17] (图 1)。例如,微生物进行硫酸盐还原时要求氧化还原电位Eh低于–100 mV[18],而产甲烷菌只有在氧化还原电位小于–330 mV的条件下才能产甲烷[19]。
|
| 图 1 地下水环境中主要微生物功能分区 Figure 1 The different ecological niches in groundwater. From the recharge zone to discharge zone of groundwater, with Eh decreasing, the function of the dominated microbial communities changed in turn of organic decomposition, nitrate reduction, iron reduction, sulfate reduction and methane production. |
1.2 地下水微生物介导的地球化学元素循环
地下水中微生物介导多种元素的地球化学循环,包括氮、碳、铁、硫和砷等。这些元素循环之间也相互影响。如碳循环过程中的厌氧甲烷氧化过程与氮循环中的硝酸盐还原耦合[20-25];化能厌氧铁氧化过程也需要硝酸盐的参与[26-27];而厌氧氨氧化过程中产生大量电子,也可以与其它元素的还原反应如铁还原耦合,即厌氧铁还原氨氧化过程(Fe reduction coupled with ammonium oxidization,Feammox)[28-30];硫酸盐还原过程中也会耦合铁和砷还原等[31-33]。
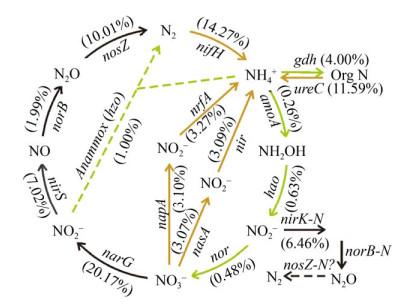
地下水系统是地球氮素循环的源和汇。近年来学界相关的研究主要集中在地下水系统氮的来源与转化过程,以及地下水系统对全球氮循环的贡献等方面。研究发现微生物矿化产氨与硝酸盐异化产氨(dissimilatory nitrate reduction to ammonium,DNRA)以及地表氮输入是高氨地下水形成的主要成因[34-35],微生物固氮也可能是高氨地下水形成的重要原因[36-37] (图 2)。此外,厌氧氨氧化(anaerobic ammonia oxidation,Anammox)是近年来地下水氮循环中新发现的途径及研究热点[38],地下水-土壤界面是Anammox反应热区,Anammox菌群是地下含水层氮素衰减的主要推手[39-40]。大量研究表明反硝化菌在地下水氮循环中起重要作用,反硝化过程可能贡献地下水中溶解N2的25%[41-45]。介导地下水中好氧氨氧化作用的菌群受氨浓度影响较大,在低氨地下水中以好氧氨氧化古菌为主,而在高氨地下水中好氧氨氧化细菌占主导地位[46-47]。
|
| 图 2 高砷地下水中氮循环基因的相对变化[36] Figure 2 The relative change of detected nitrogen cycling genes from the high arsenic group samples[36]. Arrows in orange indicate ammonium source processes, arrows in green indicate ammonium utilization processes. Percentages indicate the normalized total intensity of the functional genes in groundwaters. |
目前,人们对地下水系统中微生物介导的碳循环过程的研究主要集中在碳固定、有机质降解和产甲烷等。在自然界中已发现的6种微生物固碳途径中,目前地下水系统中发现了4种,包括卡尔文循环、还原型三羧酸循环、还原乙酰辅酶A途径和3-羟基丙酸循环/4-羟基丁酸循环[48-51]。有研究发现地下水中有机物的微生物降解可促进铁的氢氧化物还原溶解,进而促进固相中砷的释放[52],而微生物介导的产甲烷过程也可能与砷的迁移转化密切相关[53]。此外,有研究表明地下水-地表水混合区域是微生物有机碳降解的热区,微生物的异养呼吸作用改变了该区域的有机碳组成[54]。
地下水环境中也普遍存在铁循环过程,它可以控制pH值以及营养物和污染物的流动性[55-56]。如有研究发现地下水环境中含有大量的铁氧化细菌参与成矿[57-58];另外,地下水环境中还发现并分离出多种铁还原菌,这些微生物有不同的代谢特征[59]。如1株在1.7 km深的地下水中分离获得的发酵型铁还原菌Tepidibacillus decaturensis,能以H2或有机碳作为电子供体,还原铁等多种金属元素[60]。而从高砷地下水中分离得到的几株厌氧呼吸型铁还原菌——克雷伯氏菌属(Klebsiella)和希瓦氏菌属(Shewanella),能以葡萄糖为电子供体还原铁,同时导致砷的迁移;这些铁还原菌由于分泌的胞外聚合物(EPS)组分不同而释放含砷矿物中的砷,或二次成矿再次固定砷[61]。
硫酸盐还原菌(sulfate reducing bacteria,SRB)是一种通过异化作用将硫酸盐作为电子受体进行硫酸盐还原的严格厌氧菌,地下水低温低氧的特殊环境为SRB提供了适宜的生长环境。目前地下水中已发现多种SRB类群,如脱硫肠状菌属(Desulfotomaculum),脱硫球茎菌属(Desulfobulbus),脱硫八叠球菌属(Desulfosarcina)和脱硫橄榄状菌属(Desulfobacca),这些菌群通常具有较低丰度[62]。但有研究发现在硫酸盐污染的地下水中,SRB在原核生物群落中占有明显优势[63]。已有研究表明,硫酸盐、有机碳和铁等元素可促进地下水中SRB菌群的活性,进而形成了生物硫铁矿纳米颗粒[64]。另外,有研究发现地下深部微生物还可参与硫的氧化过程[65]。
砷(As)是一种有毒污染物,广泛存在于全球范围内的地下水环境中,对人类健康造成威胁[66-67]。在富砷水环境中,许多微生物被发现耐砷和/或利用砷进行呼吸代谢,能介导砷的还原、氧化、甲基化和去甲基化等过程[68-72]。这些微生物对不同碳源表现出不同的代谢能力,对砷的生物地球化学循环起重要作用[73]。目前已在地下水环境中发现了多种砷氧化还原微生物,包括副梭菌(Paraclostridium)、柠檬酸杆菌属(Citrobacter)、克雷伯氏菌属(Klebsiella)和芽孢杆菌属(Bacillus)等[74-77]。
微生物介导的地下水系统各元素循环之间常常相互影响,协同发生。如人们对硫、铁和砷的循环过程研究发现:微生物介导的硫酸盐还原促进了地下水系统中含砷铁氧化物的非生物还原,硫酸盐还原作用对含水层中铁循环和砷迁移过程起关键作用[78];沉积物中释放的砷还可以通过与硫酸盐还原作用形成硫化铁矿物共沉淀或被吸附固定[79]。最近也有研究表明,地下水系统中Feammox作用可能是导致砷释放到地下水中的重要过程[80-81]。地下水中微生物介导的碳循环过程也被发现和其它元素地球化学过程相耦合,如有研究发现地下水沉积物衍生的溶解性腐殖酸可以通过电子穿梭促进铁还原,进而影响砷迁移[82]。同时,有机碳作为微生物代谢的主要能量来源,可通过增强微生物活性来促进铁的氢氧化物还原性溶解,从而导致砷释放[83-86]。
1.3 地下水污染与修复中的生物地球化学过程地下水污染问题日益严重,除石油烃等有机污染物外,地下水污染还包括铜、铁、砷、锰、锌等重/类金属污染[87]。由于地下水污染整体特征表现为污染源多、污染面广、污染途径隐蔽和污染源滞后等[88-89],再加上地下水的埋藏性和系统复杂性,使得地下水污染防治和修复工作困难重重。早期的异位修复或物理化学修复方法成本高,困难大,因此微生物修复逐渐成为现在的研究热点。微生物修复技术主要通过地下水微生物对污染物的降解和固定来实现地下水污染物的去除,主要包括原位修复法和生物反应器法。如Michalsen等[90]采用原位修复法成功使用两株菌I-C和KTR9将地下水中的环三次甲基三硝基胺快速降解;Zhang等[91]利用S(0)或Fe(0)自养生物和异养微生物之间的生物氧化去除地下水中的钒(V);Gibert等[92]利用渗透反应屏障(PRB)在原位去除地下水中的硝酸盐,通过PRB中的活性物质为反硝化细菌提供碳源,成功地去除地下水中浓度高达280 mg/L的NO3–。由于地下水流速低,隔水层渗透性差,这使得微生物原位修复和PRB修复法容易受到制约。而采用地下循环井的方法则可增强地下水的流动性,进而大大促进微生物修复效率。如Pierro等[93]在修复氯代烃污染的地下水过程中,使用循环井加快了微生物所需碳源(3-羟基丁酸盐)的输送,极大程度地促进了修复效果。
1.4 地下水生物地球化学过程的数值模拟地下水环境的复杂性,以及水文地质过程的非线性,使得地下水中的生物地球化学过程极其复杂,实验室条件下难以模拟,因此数值模拟作为一种定量化且经济的方法被应用到地下水生物地球化学过程的研究中。利用数值模拟的方法对微生物作用进行评估,并且预测其功能和过程,已逐渐成为近年来相关研究的热点[94]。早在20世纪80年代,有研究就提出了关于潜流带生物地球化学过程的数学模型,如OTIS (One-dimensional transport with inflow and storage)模型、RTD (residence time distribution)模型、ASP (advective storage path)模型、TSM (transient storage model)模型等[95-97]。之后,越来越多的地下水、地表水数值模型也被应用到研究中。如Shapiro等[98]利用基因组数据预测自然环境中微生物代谢速率,通过模拟含水层中产甲烷菌预测该类菌体代谢过程中的生化反应速率,明确跟踪了产甲烷过程中碳和能量的细胞通量。Shi等[99]建立了微生物介导砷还原和铁氧化物转化的耦合动力学模型,并根据砷还原基因arrA的表达模式对砷还原速率进行了量化。又如Lai等[100]建立了数学模型来评估纳米零价铁(NZVI)微生物脱氮去除地下水硝酸盐技术的性能。Valsala等[101]通过建立有限差分模型的数值模拟方法,探究了胶体和微生物共存对地下水中BTEX (苯、甲苯、乙苯、二甲苯)迁移的影响。目前,微生物数值模拟方面的研究还处于初步阶段,大量的地下水“微生物暗过程”还有待采用数值模拟的办法进行预测和探究。
2 问题和展望随着(宏)基因/转录组学、代谢组学、宏表型组学等技术的快速发展以及各种分析手段的不断革新,学界对地下水系统中生物地球化学循环的了解更加全面深入,同时也对地下水微生物功能群及生物地球化学循环研究提出更高的要求。未来地下水微生物功能群及生物地球化学循环的研究亟待围绕以下几个方面展开。
地下水微生物介导的元素地球化学循环过程亟待进一步深入,这些过程中的能量转换和代谢新途径及其调控机制还有待大量深入研究。随着各种研究手段的革新,围绕地下水中微生物作用的微观机制及其宏观生态效应,深入探索地下水微生物介导的元素地球化学循环过程中多元素多过程的耦合关系,以及它们在地球“水圈”、“岩石圈”和“大气圈”中作用的研究将成为未来的热点。
修复理论方法和技术创新将成为地下水可持续安全供给的基石。地下水修复的生物地球化学理论研究、生物技术应用研究及生物修复工程化方面还比较薄弱,还缺乏对地下水微生物功能群演化和生物地球化学过程的完整识别。另外由于地下水环境不同于地表,复杂多变且不可见,地下水修复微生物功能群及其地球化学过程的原位检测和野外长期监测是难点。未来地下水修复的重点研究方向需要多头并进:开发原位模拟方法和长期监测技术,微生物修复与植物修复、电化学修复等多技术联合。
地下水与人类生活关系密切,直接影响人类身体健康。因此,全面深入理解地下水微生物作用与地质成因和人为活动影响下各种致畸致癌等有害物质的迁移转化过程及其健康风险,对保证国家供水安全和维护公共健康起着十分重要的作用。近年来医学地质学的提出将面临新的重大社会需求,人类在谋求人与自然和谐发展的进程中,“同一健康”概念(one health concept)下的地下水医学地质学的重要性将与日俱增[102],将是今后的一个重要研究方向。
微生物是地球上最丰富多样的细胞生命形式,占据了所有可能的生态位。而绝大部分微生物都不能通过纯培养获得,即被称为“微生物暗物质”。地下水系统中大量未知的微生物类群以及“微生物暗物质”亟待学界进一步挖掘[103]。通过纯培养技术获得地下水微生物可培养信息与资源方面的研究,或通过不依赖纯培养且具有高分辨率的单细胞技术,如纳米二次离子质谱(NanoSIMS)和单细胞拉曼光谱等获得微生物细胞共代谢信息,将是今后的又一个重要研究方向[104-106]。
随着科学技术的发展,分子生物学、多组学、生物信息学、同位素地球化学、微区地球化学分析、高分辨率电镜技术、数值模拟等多种技术方法、多学科的交叉融合将应用到地下水微生物的研究中,这些必将成为今后研究的有力工具,在很大程度上拓展我们对地下水认知的深度和广度,为地下水科学的发展以及地下水的安全供给提供广阔空间。
| [1] |
Lin XY. Historical change and prospect of discipline develop ent of "groundwater science and engineering". Journal of Jilin University: Earth Science Edition, 2007, 37(2): 209-215.
(in Chinese) 林学钰. "地下水科学与工程"学科形成的历史沿革及其发展前景. 吉林大学学报: 地球科学版, 2007, 37(2): 209-215. |
| [2] | John DE, Rose JB. Review of factors affecting microbial survival in groundwater. Environmental Science Technology, 2005, 39(19): 7345-7356. DOI:10.1021/es047995w |
| [3] | Griebler C, Lueders T. Microbial biodiversity in groundwater ecosystems. Freshwater Biology, 2009, 54(4): 649-677. DOI:10.1111/j.1365-2427.2008.02013.x |
| [4] |
He XY, Chen Y. Microbes and their purification effect in groundwater. Studies of Trace Elements and Health, 2015, 32(5): 56-59.
(in Chinese) 何湘云, 陈烨. 地下水中的微生物及其净化作用. 微量元素与健康研究, 2015, 32(5): 56-59. |
| [5] | Anantharaman K, Brown CT, Hug LA, Sharon I, Castelle CJ, Probst AJ, Thomas BC, Singh A, Wilkins MJ, Karaoz U, Brodie EL, Williams KH, Hubbard SS, Banfield JF. Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nature Communications, 2016, 7(1): 13219-13219. DOI:10.1038/ncomms13219 |
| [6] | Moore A, Lenczewski M, Leal-Bautista RM, Duvall M. Groundwater microbial diversity and antibiotic resistance linked to human population density in Yucatan Peninsula, Mexico. Canadian Journal of Microbiology, 2020, 66(1): 46-58. DOI:10.1139/cjm-2019-0173 |
| [7] | Savio D, Stadler P, Reischer GH, Demeter K, Linke RB, Blaschke AP, Mach RL, Kirschner AKT, Stadler H, Farnleitner AH. Spring water of an alpine Karst aquifer is dominated by a taxonomically stable but discharge-responsive bacterial community. Frontiers in Microbiology, 2019, 10: 28. DOI:10.3389/fmicb.2019.00028 |
| [8] | Sonthiphand P, Ruangroengkulrith S, Mhuantong W, Charoensawan V, Chotpantarat S, Boonkaewwan S. Metagenomic insights into microbial diversity in a groundwater basin impacted by a variety of anthropogenic activities. Environmental Science and Pollution Research, 2019, 26(26): 26765-26781. DOI:10.1007/s11356-019-05905-5 |
| [9] | Schwab VF, Herrmann M, Roth V, Gleixner G, Lehmann R, Pohnert G, Trumbore SE, Kusel K, Totsche KU. Functional diversity of microbial communities in pristine aquifers inferred by PLFA- and sequencing-based approaches. Biogeosciences, 2016, 14(10): 2697-2714. |
| [10] | Jiang Z, Li P, Wang YH, Liu H, Wei DZ, Yuan CG, Wang HL. Arsenic mobilization in a high arsenic groundwater revealed by metagenomic and Geochip analyses. Scientific Reports, 2019, 9(1): 12972. DOI:10.1038/s41598-019-49365-w |
| [11] | Chapelle FH. The significance of microbial processes in hydrogeology and geochemistry. Hydrogeology Journal, 2000, 8(1): 41-46. DOI:10.1007/PL00010973 |
| [12] |
Guo H, Ni P, Jia Y, Guo Q, Jiang Y. Types, chemical characteristics and genesis of geogenic high-arsenic groundwater in the world. Earth Science Frontiers, 2014, 21(4): 1-12.
(in Chinese) 郭华明, 倪萍, 贾永锋, 郭琦, 姜玉肖. 原生高砷地下水的类型、化学特征及成因. 地学前缘, 2014, 21(4): 1-12. |
| [13] |
He X, Ma T, Wang YX, Deng YM, Huang B, He J, Zhao J, Tian CY, Li ZL. Geochemical characteristics of the As-bearing aquifer in the Hetao plain, Inner Mongolia. Chinese Geology, 2010, 37(3): 781-788.
(in Chinese) 何薪, 马腾, 王焰新, 邓娅敏, 黄彬, 何军, 赵洁, 田春艳, 李振龙. 内蒙古河套平原高砷地下水赋存环境特征. 中国地质, 2010, 37(3): 781-788. DOI:10.3969/j.issn.1000-3657.2010.03.034 |
| [14] |
Zhang FC, Wen DG, Guo JQ, Zhang EY, Hao AB, An YH. Research progress and prospect of geological environment in main endemic disease area. Chinese Geology, 2010, 37(3): 551-562.
(in Chinese) 张福存, 文冬光, 郭建强, 张二勇, 郝爱兵, 安永会. 中国主要地方病区地质环境研究进展与展望. 中国地质, 2010, 37(3): 551-562. DOI:10.3969/j.issn.1000-3657.2010.03.002 |
| [15] |
Zhang LP, Xie XJ, Li JX, Wang YX. Spatial variation, speciation and enrichment of arsenic in groundwater from the Datong basin, Northern China. Geological Science and Technology Information, 2014, 33(1): 178-184.
(in Chinese) 张丽萍, 谢先军, 李俊霞, 王焰新. 大同盆地地下水中砷的形态、分布及其富集过程研究. 地质科技情报, 2014, 33(1): 178-184. |
| [16] | Shamsudduha M, Marzen LJ, Uddin A, Lee MK, Saunders JA. Spatial relationship of groundwater arsenic distribution with regional topography and water-table fluctuations in the shallow aquifers in Bangladesh. Environmental Geology, 2008, 57(7): 1521-1535. DOI:10.1007/s00254-008-1429-3 |
| [17] | Xie X, Ellis A, Wang Y, Xie Z, Duan M, Su C. Geochemistry of redox-sensitive elements and sulfur isotopes in the high arsenic groundwater system of Datong Basin, China. Science of the Total Environment, 2009, 407(12): 3823-3835. DOI:10.1016/j.scitotenv.2009.01.041 |
| [18] | Postgate JR. The sulfate-reducing bacteria. Cambridge: Cambridge University Press, 1965. |
| [19] | Jee HS, Nishio N. Influence of redox potential on biomethanation of H2 and CO2 by methanobacterium thermoautotrophicum in Eh-Sat batch cultures. Journal of Genaral and Applied Microbiology, 1987, 33(5): 401-409. DOI:10.2323/jgam.33.401 |
| [20] | Luo J, Chen H, Yuan Z, Guo J. Methane-supported nitrate removal from groundwater in a membrane biofilm reactor. Water Research, 2018, 132: 71-78. DOI:10.1016/j.watres.2017.12.064 |
| [21] | Zhang B, Jiang Y, Zuo K, He C, Dai Y, Ren ZJ. Microbial vanadate and nitrate reductions coupled with anaerobic methane oxidation in groundwater. Journal of Hazardous Materials, 2020, 382: 121228. DOI:10.1016/j.jhazmat.2019.121228 |
| [22] | Shen L, Tian M, Cheng H, Liu X, Yang Y, Liu J, Xu J, Kong Y, Li J, Liu Y. Different responses of nitrite-and nitrate-dependent anaerobic methanotrophs to increasing nitrogen loading in a freshwater reservoir. Environmental Pollution, 2020, 263: 114623. DOI:10.1016/j.envpol.2020.114623 |
| [23] | Haroon MF, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan Z, Tyson GW. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature, 2013, 500(7464): 567-570. DOI:10.1038/nature12375 |
| [24] | Jiang LP, Hu Z, Wang YN, Ru DY, Li JW, Fan JL. Effect of trace elements on the development of co-cultured nitrite-dependent anaerobic methane oxidation and methanogenic bacteria consortium. Bioresource Technology, 2018, 268: 190-196. DOI:10.1016/j.biortech.2018.07.139 |
| [25] | van Grinsven S, Sinninghe Damsté JS, Abdala Asbun A, Engelmann JC, Harrison J, Villanueva L. Methane oxidation in anoxic lake water stimulated by nitrate and sulfate addition. Environmental Microbiology, 2020, 22(2): 766-782. DOI:10.1111/1462-2920.14886 |
| [26] | Zhang J, Chai CW, ThomasArrigo LK, Zhao SC, Kretzschmar R, Zhao FJ. Nitrite accumulation is required for microbial anaerobic iron oxidation, but not for arsenite oxidation, in two heterotrophic denitrifiers. Environmental Science & Technology, 2020, 54(7): 4036-4045. |
| [27] | Su JF, Shao SC, Huang T, Ma F, Yang S F, Zhou ZM, Zheng SC. Anaerobic nitrate-dependent iron(Ⅱ) oxidation by a novel autotrophic bacterium, Pseudomonas sp. SZF15. Journal of environmental chemical engineering, 2015, 3(3): 2187-2193. DOI:10.1016/j.jece.2015.07.030 |
| [28] | Ding B, Chen Z, Li Z, Qin Y, Chen S. Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from ecosystem habitats in the Taihu estuary region. Science of The Total Environment, 2019, 662: 600-606. DOI:10.1016/j.scitotenv.2019.01.231 |
| [29] | Shuai WT, Jaffé PR. Anaerobic ammonium oxidation coupled to iron reduction in constructed wetland mesocosms. The Science of the Total Environment, 2019, 648: 984-992. DOI:10.1016/j.scitotenv.2018.08.189 |
| [30] | Ding BJ, Li ZK, Qin YB. Nitrogen loss from anaerobic ammonium oxidation coupled to iron(Ⅲ) reduction in a riparian zone. Environmental Pollution: Barking, Essex, 2017, 231(Pt 1): 379-386. |
| [31] | Reyes C, Schneider D, Thürmer A, Kulkarni A, Lipka M, Sztejrenszus SY, Böttcher ME, Daniel R, Friedrich MW. Potentially active iron, sulfur, and sulfate reducing bacteria in Skagerrak and bothnian bay sediments. Geomicrobiology Journal, 2017, 34(10): 840-850. DOI:10.1080/01490451.2017.1281360 |
| [32] | Sun HF, Shi BY, Yang F, Wang DS. Effects of sulfate on heavy metal release from iron corrosion scales in drinking water distribution system. Water Research, 2017, 114: 69-77. DOI:10.1016/j.watres.2017.02.021 |
| [33] | Muller JB, Ramos DT, Larose C, Fernandes M, Lazzarin HS, Vogel TM, Corseuil HX. Combined iron and sulfate reduction biostimulation as a novel approach to enhance BTEX and PAH source-zone biodegradation in biodiesel blend-contaminated groundwater. Journal of Hazardous Materials, 2017, 326: 229-236. DOI:10.1016/j.jhazmat.2016.12.005 |
| [34] | Liang Y, Ma R, Wang Y, Wang S, Qu L, Wei W, Gan Y. Hydrogeological controls on ammonium enrichment in shallow groundwater in the central Yangtze River Basin. Science of The Total Environment, 2020, 741: 140350. DOI:10.1016/j.scitotenv.2020.140350 |
| [35] | Du Y, Deng YM, Ma T, Xu Y, Tao YQ, Huang YW, Liu R, Wang YX. Enrichment of geogenic ammonium in quaternary alluvial-lacustrine aquifer systems: evidence from carbon isotopes and DOM characteristics. Environmental Science & Technology, 2020, 54(10): 6104-6114. |
| [36] | Li P, Jiang Z, Wang YH, Deng Y, van Nostrand JD, Yuan T, Liu H, Wei DZ, Zhou JZ. Analysis of the functional gene structure and metabolic potential of microbial community in high arsenic groundwater. Water Research, 2017, 123: 268-276. DOI:10.1016/j.watres.2017.06.053 |
| [37] | Méheust R, Castelle CJ, Matheus Carnevali PB, Farag IF, He C, Chen LX, Amano Y, Hug LA, Banfield JF. Groundwater Elusimicrobia are metabolically diverse compared to gut microbiome Elusimicrobia and some have a novel nitrogenase paralog. The ISME Journal, 2020, 14(12): 2907-2922. DOI:10.1038/s41396-020-0716-1 |
| [38] | Gao DW, Wang XL, Liang H, Wei QH, Dou Y, Li LW. Anaerobic ammonia oxidizing bacteria: ecological distribution, metabolism, and microbial interactions. Frontiers of Environmental Science & Engineering, 2018, 12(3): 1-15. DOI:10.1007/s11783-018-1035-x |
| [39] | Wang Y, Xu LY, Wang SY, Ye F, Zhu G B. Global distribution of anaerobic ammonia oxidation (Anammox) bacteria-field surveys in wetland, dryland, groundwater aquifer and snow. Frontiers in Microbiology, 2019, 10: 2583. DOI:10.3389/fmicb.2019.02583 |
| [40] | Wang SY, Zhu GB, Zhuang LJ, Li YX, Liu L, Lavik G, Berg M, Liu ST, Long XE, Guo JH, Jetten MSM, Kuypers MMM, Li FB, Schwark L, Yin CQ. Anaerobic ammonium oxidation is a major N-sink in aquifer systems around the world. The ISME Journal, 2020, 14(1): 151-163. DOI:10.1038/s41396-019-0513-x |
| [41] | Kumar S, Herrmann M, Blohm A, Hilke I, Frosch T, Trumbore SE, Küsel K. Thiosulfate-and hydrogen-driven autotrophic denitrification by a microbial consortium enriched from groundwater of an oligotrophic limestone aquifer. FEMS Microbiology Ecology, 2018, 94(10). |
| [42] | Kim H, Kaown D, Mayer B, Lee J, Hyun Y, Lee K. Identifying the sources of nitrate contamination of groundwater in an agricultural area (Haean basin, Korea) using isotope and microbial community analyses. Science of The Total Environment, 2015, 533: 566-575. DOI:10.1016/j.scitotenv.2015.06.080 |
| [43] | Safonov AV, Babich TL, Sokolova DS, Grouzdev DS, Tourova TP, Poltaraus AB, Zakharova EV, Merkel AY, Novikov AP, Nazina TN. Microbial community and in situ bioremediation of groundwater by nitrate removal in the zone of a radioactive waste surface repository. Frontiers in Microbiology, 2018, 9(1985): 1985. |
| [44] | Amo EHD, Mencio A, Gich F, Mas-Pla J, Baneras L. Isotope and microbiome data provide complementary information to identify natural nitrate attenuation processes in groundwater. Science of The Total Environment, 2018, 613: 579-591. |
| [45] | Liu Y, Feng C P, Chen N, Sheng YZ, Dong SS, Hao CB, Lei K. Bioremediation of nitrate and Fe(Ⅱ) combined contamination in groundwater by heterotrophic denitrifying bacteria and microbial community analysis. RSC Advances, 2016, 6(110): 108375-108383. DOI:10.1039/C6RA22687F |
| [46] | Lee KH, Wang YF, Wang Y, Gu JD, Jiao JJ. Abundance and diversity of aerobic/anaerobic ammonia/ammonium-oxidizing microorganisms in an ammonium-rich aquitard in the Pearl River Delta of South China. Microbial Ecology, 2018, 76(1): 81-91. DOI:10.1007/s00248-016-0815-8 |
| [47] | Wang H, Li P, Liu H, Tan T, Yang G, Zhang R. Microorganisms for ammonia/ammonium-oxidization and anammox detected in Holocene-Pleistocene aquifers with high arsenic concentrations. International Biodeterioration and Biodegradation, 2021, 157: 105136. DOI:10.1016/j.ibiod.2020.105136 |
| [48] | Alfreider A, Vogt C. Genetic evidence for bacterial chemolithoautotrophy based on the reductive tricarboxylic acid cycle in groundwater systems. Microbes and Environments, 2012, 27(2): 209-214. DOI:10.1264/jsme2.ME11274 |
| [49] | Alfreider A, Vogt C, Hoffmann D, Babel W. Diversity of ribulose-1, 5-bisphosphate carboxylase/oxygenase large-subunit genes from groundwater and aquifer microorganisms. Microbial Ecology, 2003, 45(4): 317-328. DOI:10.1007/s00248-003-2004-9 |
| [50] | Wegner CE, Gaspar M, Geesink P, Herrmann M, Marz M, Kusel K. Biogeochemical regimes in shallow aquifers reflect the metabolic coupling of the elements nitrogen, sulfur, and carbon. Applied and Environmental Microbiology, 2019, 85(5). |
| [51] | Seyler LM, Brazelton WJ, McLean C, Putman LI, Hyer A, Kubo MDY, Hoehler T, Cardace D, Schrenk MO. Carbon assimilation strategies in ultrabasic groundwater: clues from the integrated study of a serpentinization-influenced aquifer. bioRxiv, 2019. DOI:10.1101/776849 |
| [52] | Qiao W, Guo HM, He C, Shi Q, Xiu W, Zhao B. Molecular evidence of arsenic mobility linked to biodegradable organic matter. Environmental Science & Technology, 2020, 54(12): 7280-7290. |
| [53] | Wang YH, Li P, Dai XY, Zhang R, Jiang Z, Jiang DW, Wang YX. Abundance and diversity of methanogens: potential role in high arsenic groundwater in Hetao Plain of Inner Mongolia, China. The Science of the Total Environment, 2015, 515/516: 153-161. DOI:10.1016/j.scitotenv.2015.01.031 |
| [54] | Stegen JC, Fredrickson JK, Wilkins MJ, Konopka A, Nelson WC, Arntzen EV, Chrisler WB, Chu RK, Danczak RE, Fansler SJ. Groundwater-surface water mixing shifts ecological assembly processes and stimulates organic carbon turnover. Nature Communications, 2016, 7(1): 11237-11237. DOI:10.1038/ncomms11237 |
| [55] | Jambor JL, Dutrizac JE. Occurrence and constitution of natural and synthetic ferrihydrite, a widespread iron oxyhydroxide. Chemical Reviews, 1998, 98(7): 2549-2586. DOI:10.1021/cr970105t |
| [56] | Blowes DW, Ptacek CJ, Jambor JL, Weisener CG. The geochemistry of acid mine drainage. In: treatise on geochemistry, K.K. Turekian (Ed. ). Pergamon, Oxford, 2003: 149-204. |
| [57] | Almaraz N, Whitaker AH, Andrews MY, Duckworth OW. Assessing biomineral formation by iron-oxidizing bacteria in a circumneutral creek. Journal of Contemporary Water Research Education, 2017, 160(1): 60-71. DOI:10.1111/j.1936-704X.2017.03240.x |
| [58] | Edwards BA, Shirokova VL, Enright AML, Ferris FG. Dependence of in situ bacterial Fe(Ⅱ)-oxidation and Fe(Ⅲ)-precipitation on sequential reactive transport. Geomicrobiology Journal, 2018, 35(6): 503-510. DOI:10.1080/01490451.2017.1394929 |
| [59] | Dai X, Li P, Tu J, Zhang R, Wei D, Li B, Wang Y, Jiang Z. Evidence of arsenic mobilization mediated by an indigenous iron reducing bacterium from high arsenic groundwater aquifer in Hetao Basin of Inner Mongolia, China. International Biodeterioration Biodegradation, 2016, 128: 22-27. |
| [60] | Dong Y, Sanford RA, Boyanov MI, Kemner KM, Flynn TM, Oloughlin EJ, Locke RA, Weber JR, Egan SM, Fouke BW. Tepidibacillus decaturensis sp. nov., a microaerophilic, moderately thermophilic iron-reducing bacterium isolated from 1.7 km depth groundwater. International Journal of Systematic Evolutionary Microbiology, 2016, 66(10): 3964-3971. DOI:10.1099/ijsem.0.001295 |
| [61] | Liu H, Li P, Wang H, Qing C, Tan T, Shi B, Zhang G, Jiang Z, Wang Y, Hasan SZ. Arsenic mobilization affected by extracellular polymeric substances (EPS) of the dissimilatory iron reducing bacteria isolated from high arsenic groundwater. Science of The Total Environment, 2020, 735: 139501. DOI:10.1016/j.scitotenv.2020.139501 |
| [62] | Li P, Li B, Webster G, Wang YH, Jiang DW, Dai XY, Jiang Z, Dong HL, Wang YX. Abundance and diversity of sulfate-reducing bacteria in high arsenic shallow aquifers. Geomicrobiology Journal, 2014, 31(9): 802-812. DOI:10.1080/01490451.2014.893181 |
| [63] | An XL, Baker P, Li H, Su JQ, Yu CP, Cai C. The patterns of bacterial community and relationships between sulfate-reducing bacteria and hydrochemistry in sulfate-polluted groundwater of Baogang rare earth tailings. Environmental Science and Pollution Research, 2016, 23(21): 21766-21779. DOI:10.1007/s11356-016-7381-y |
| [64] | Saunders JA, Lee M, Dhakal P, Ghandehari SS, Wilson T, Billor MZ, Uddin A. Bioremediation of arsenic-contaminated groundwater by sequestration of arsenic in biogenic pyrite. Applied Geochemistry, 2018, 96: 233-243. DOI:10.1016/j.apgeochem.2018.07.007 |
| [65] | Bell E, Lamminmäki T, Alneberg J, Andersson AF, Qian C, Xiong WL, Hettich RL, Frutschi M, Bernier-Latmani R. Active sulfur cycling in the terrestrial deep subsurface. The ISME Journal, 2020, 14(5): 1260-1272. DOI:10.1038/s41396-020-0602-x |
| [66] | Nordstrom DK. Worldwide occurrences of arsenic in ground water. Science, 2002, 296(5576): 2143-2145. DOI:10.1126/science.1072375 |
| [67] | Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 2002, 17(5): 517-568. DOI:10.1016/S0883-2927(02)00018-5 |
| [68] | Huang JH. Impact of microorganisms on arsenic biogeochemistry: a review. Water, Air, & Soil Pollution, 2014, 225(2): 1-25. DOI:10.1007/s11270-013-1848-y |
| [69] | Oremland RS, Stolz JF. The ecology of arsenic. Science, 2003, 300(5621): 939-944. DOI:10.1126/science.1081903 |
| [70] | Wang YH, Li P, Jiang Z, Liu H, Wei DZ, Wang HL, Wang YX. Diversity and abundance of arsenic methylating microorganisms in high arsenic groundwater from Hetao Plain of Inner Mongolia, China. Ecotoxicology: London, England, 2018, 27(8): 1047-1057. DOI:10.1007/s10646-018-1958-9 |
| [71] | Biswas R, Sarkar A. Characterization of arsenite-oxidizing bacteria to decipher their role in arsenic bioremediation. Preparative Biochemistry & Biotechnology, 2019, 49(1): 30-37. |
| [72] | Hamontree C, Pipattanajaroenkul P, Sonthiphand P, Kraidech S, Boonkaewwan S, Chotpantarat S. Detection of arsenite-oxidizing bacteria in groundwater with low arsenic concentration in Rayong province, Thailand. MATEC Web of Conferences, 2018, 192: 3036. DOI:10.1051/matecconf/201819203036 |
| [73] | 王培培. 厌氧微生物砷甲基转移酶的基因克隆及作用机制研究. 中国科学院大学学位论文, 2014. |
| [74] | Tian HX, Wang J, Li JY, Wang YJ, Mallavarapu M, He WX. Six new families of aerobic arsenate reducing bacteria: Leclercia, Raoultella, Kosakonia, Lelliottia, Yokenella, and Kluyvera. Geomicrobiology Journal, 2019, 36(4): 339-347. DOI:10.1080/01490451.2018.1554726 |
| [75] | Mohapatra B, Sarkar A, Joshi S, Chatterjee A, Kazy SK, Maiti MK, Satyanarayana T, Sar P. An arsenate-reducing and alkane-metabolizing novel bacterium, Rhizobium arsenicireducens sp. nov., isolated from arsenic-rich groundwater. Archives of Microbiology, 2017, 199(2): 191-201. DOI:10.1007/s00203-016-1286-5 |
| [76] | Biswas R, Majhi AK, Sarkar A. The role of arsenate reducing bacteria for their prospective application in arsenic contaminated groundwater aquifer system. Biocatalysis Agricultural Biotechnology, 2019, 20: 101218. DOI:10.1016/j.bcab.2019.101218 |
| [77] | Wang YH, Wei DZ, Li P, Jiang Z, Liu H, Qing C, Wang HL. Diversity and arsenic-metabolizing gene clusters of indigenous arsenate-reducing bacteria in high arsenic groundwater of the Hetao Plain, Inner Mongolia. Ecotoxicology, 2020: 1-9. DOI:10.1007/s10646-020-02305-1 |
| [78] | Zheng TL, Deng YM, Wang YX, Jiang HC, Xie XJ, Gan YQ. Microbial sulfate reduction facilitates seasonal variation of arsenic concentration in groundwater of Jianghan Plain, Central China. The Science of the Total Environment, 2020, 735: 139327. DOI:10.1016/j.scitotenv.2020.139327 |
| [79] | Deng YM, Zheng TL, Wang YX, Liu L, Jiang HC, Ma T. Effect of microbially mediated iron mineral transformation on temporal variation of arsenic in the Pleistocene aquifers of the central Yangtze River basin. The Science of the Total Environment, 2018, 619/620: 1247-1258. DOI:10.1016/j.scitotenv.2017.11.166 |
| [80] | Weng TN, Liu CW, Kao YH, Hsiao SSY. Isotopic evidence of nitrogen sources and nitrogen transformation in arsenic-contaminated groundwater. The Science of the Total Environment, 2017, 578: 167-185. DOI:10.1016/j.scitotenv.2016.11.013 |
| [81] | Xiu W, Lloyd J, Guo HM, Dai W, Nixon S, Bassil NM, Ren C, Zhang CR, Ke TT, Polya D. Linking microbial community composition to hydrogeochemistry in the western Hetao Basin: potential importance of ammonium as an electron donor during arsenic mobilization. Environment International, 2020, 136: 105489. DOI:10.1016/j.envint.2020.105489 |
| [82] | Kulkarni HV, Mladenov N, McKnight DM, Zheng Y, Kirk MF, Nemergut DR. Dissolved fulvic acids from a high arsenic aquifer shuttle electrons to enhance microbial iron reduction. The Science of the Total Environment, 2018, 615: 1390-1395. DOI:10.1016/j.scitotenv.2017.09.164 |
| [83] | Lawson M, Polya DA, Boyce AJ, Bryant CL, Ballentine CJ. Tracing organic matter composition and distribution and its role on arsenic release in shallow Cambodian groundwaters. Geochimica et Cosmochimica Acta, 2016, 178: 160-177. DOI:10.1016/j.gca.2016.01.010 |
| [84] | Campbell KM, Malasarn D, Saltikov CW, Newman DK, Hering JG. Simultaneous microbial reduction of iron(Ⅲ) and arsenic(Ⅴ) in suspensions of Hydrous ferric oxide. Environmental Science & Technology, 2006, 40(19): 5950-5955. |
| [85] | Guo H, Tang X, Yang S, Shen Z. Effect of indigenous bacteria on geochemical behavior of arsenic in aquifer sediments from the Hetao Basin, Inner Mongolia: Evidence from sediment incubations. Applied Geochemistry, 2008, 23(12): 3267-3277. DOI:10.1016/j.apgeochem.2008.07.010 |
| [86] | Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Lloyd JR. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature, 2004, 430(6995): 68-71. DOI:10.1038/nature02638 |
| [87] |
He YX, Dai CM, Su YM, Zhang YL. Research progress of remediation technologies on heavy metal pollution in groundwater. Technology of Water Treatment, 2016, 42(2): 1-5, 26.
(in Chinese) 贺亚雪, 代朝猛, 苏益明, 张亚雷. 地下水重金属污染修复技术研究进展. 水处理技术, 2016, 42(2): 1-5, 26. |
| [88] |
Ye YT, Fang HP, Zhang MQ, Yan R. Analysis of groundwater pollution and remediation technology. Guizhou Agricultural Mechaniation, 2019(3): 22-24, 31.
(in Chinese) 叶粤婷, 方宏萍, 张美崎, 严容. 浅析地下水污染现状及修复技术. 贵州农机化, 2019(3): 22-24, 31. |
| [89] |
Zheng CQ, Zhi GQ, Li TF, Guo HL. Analysis of current situation and countermeasures of groundwater pollution in China. Yunnan Environmental Science, 2018, 37(A1): 49-52.
(in Chinese) 郑才庆, 支国强, 李田富, 郭宏龙. 我国地下水污染现状及对策措施分析. 环境科学导刊, 2018, 37(A1): 49-52. |
| [90] | Michalsen MM, King AS, Istok JD, Crocker FH, Fuller ME, Kucharzyk KH, Gander MJ. Spatially-distinct redox conditions and degradation rates following field-scale bioaugmentation for RDX-contaminated groundwater remediation. Journal of Hazardous Materials, 2020, 387: 121529. DOI:10.1016/j.jhazmat.2019.121529 |
| [91] | Zhang B, Qiu R, Lu L, Chen X, He C, Lu J, Ren ZJ. Autotrophic vanadium(Ⅴ) bioreduction in groundwater by elemental sulfur and zerovalent iron. Environmental Science Technology, 2018, 52(13): 7434-7442. DOI:10.1021/acs.est.8b01317 |
| [92] | Gibert O, Assal A, Devlin H, Elliot T, Kalin RM. Performance of a field-scale biological permeable reactive barrier for in situ remediation of nitrate-contaminated groundwater. The Science of the Total Environment, 2019, 659: 211-220. DOI:10.1016/j.scitotenv.2018.12.340 |
| [93] | Pierro L, Matturro B, Rossetti S, Sagliaschi M, Sucato S, Alesi E, Bartsch E, Arjmand F, Papini MP. Polyhydroxyalkanoate as a slow-release carbon source for in situ bioremediation of contaminated aquifers: from laboratory investigation to pilot-scale testing in the field. New Biotechnology, 2017, 37: 60-68. DOI:10.1016/j.nbt.2016.11.004 |
| [94] |
Wang CX, Su YM, Zhou XF, Zhang YL. Progress in the simulation of pore migration and reaction of pollutants in groundwater. Regional Govenance,, 2019(51): 138-142.
(in Chinese) 王慈炫, 苏益明, 周雪飞, 张亚雷. 地下水中污染物孔隙迁移-反应模拟的进展. 区域治理, 2019(51): 138-142. |
| [95] | Bencala KE. Simulation of solute transport in a mountain pool-and-riffle stream with a kinetic mass-transfer for sorption. Water Resources Research, 1983, 19(3): 732-738. DOI:10.1029/WR019i003p00732 |
| [96] | Gooseff MN, Wondzell SM, Haggerty R, Anderson J. Comparing transient storage modeling and residence time distribution (RTD) analysis in geomorphically varied reaches in the Lookout Creek basin, Oregon, USA. Advances in Water Resources, 2003, 26(9): 925-937. DOI:10.1016/S0309-1708(03)00105-2 |
| [97] | Salehin M, Packman AI, Worman A. Comparison of transient storage in vegetated and unvegetated reaches of a small agricultural stream in Sweden: seasonal variation and anthropogenic manipulation. Advances in Water Resources, 2003, 26(9): 951-964. DOI:10.1016/S0309-1708(03)00084-8 |
| [98] | Shapiro B, Hoehler TM, Jin Q. Integrating genome-scale metabolic models into the prediction of microbial kinetics in natural environments. Geochimica et Cosmochimica Acta, 2018, 242: 102-122. DOI:10.1016/j.gca.2018.08.047 |
| [99] | Shi ZQ, Hu SW, Lin JY, Liu TX, Li XM, Li FB. Quantifying microbially mediated kinetics of ferrihydrite transformation and arsenic reduction: role of the arsenate-reducing gene expression pattern. Environmental Science & Technology, 2020, 54(11): 6621-6631. |
| [100] | Peng L, Liu YW, Gao SH, Chen XM, Xin P, Dai XH, Ni BJ. Evaluation on the nanoscale zero valent iron based microbial denitrification for nitrate removal from groundwater. Scientific Reports, 2015, 5: 12331. DOI:10.1038/srep12331 |
| [101] | Valsala R, Govindarajan SK. Co-colloidal BTEX and microbial transport in a saturated porous system: numerical modeling and sensitivity analysis. Transport in Porous Media, 2019, 127(2): 269-294. DOI:10.1007/s11242-018-1191-2 |
| [102] |
Wang Y. Innovative development of medical geology: a one health perspective. Earth Science-Journal of China University of Geosciences, 2020, 45(4): 1093-1102.
(in Chinese) 王焰新. "同一健康"视角下医学地质学的创新发展. 地球科学, 2020, 45(4): 1093-1102. |
| [103] | Rinke C, Schwientek P, Sczyrba A, Ivanova NN, Anderson IJ, Cheng JF, Darling A, Malfatti S, Swan BK, Gies EA, Dodsworth JA, Hedlund BP, Tsiamis G, Sievert SM, Liu WT, Eisen JA, Hallam SJ, Kyrpides NC, Stepanauskas R, Rubin EM, Hugenholtz P, Woyke T. Insights into the phylogeny and coding potential of microbial dark matter. Nature, 2013, 499(7459): 431-437. |
| [104] | Franca L, Lopezlopez A, Rossellomora R, Costa MSD. Microbial diversity and dynamics of a groundwater and a still bottled natural mineral water. Environmental Microbiology, 2015, 17(3): 577-593. |
| [105] | Lennon JT, Jones SE. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nature Reviews Microbiology, 2011, 9(2): 119-130. |
| [106] | D'Onofrio A, Crawford JM, Stewart EJ, Witt K, Gavrish E, Epstein S, Clardy J, Lewis K. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chemistry & Biology, 2010, 17(3): 254-264. |
 2021, Vol. 61
2021, Vol. 61